- All the masses of the elements are determined relative to 12C. Average Atomic Mass Since many elements have a number of isotopes, chemists use average atomic mass. On the periodic table the mass of carbon is reported as 12.011 amu. No single carbon atom has a mass of 12.011, but in a handful of C atoms the average mass of a carbon atom is 12.011.
- Chemical elements listed by atomic mass The elements of the periodic table sorted by atomic mass. Click on any element's name for further information on chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry.
The relative atomic mass is the average mass of an atom compared to. The mass of a carbon-12 atom. 1/12 the mass of a carbon-12 atom. The mass of a hydrogen atom. 1/12 the mass of a hydrogen atom. Tags: Question 9. The relative molecular mass of a compound is equal to the sum of its.
1 u = 1.66 × 10-27 kg
We can estmate the the relative atomic mass (atomic weight) of an element E with the naturally occurring isotopes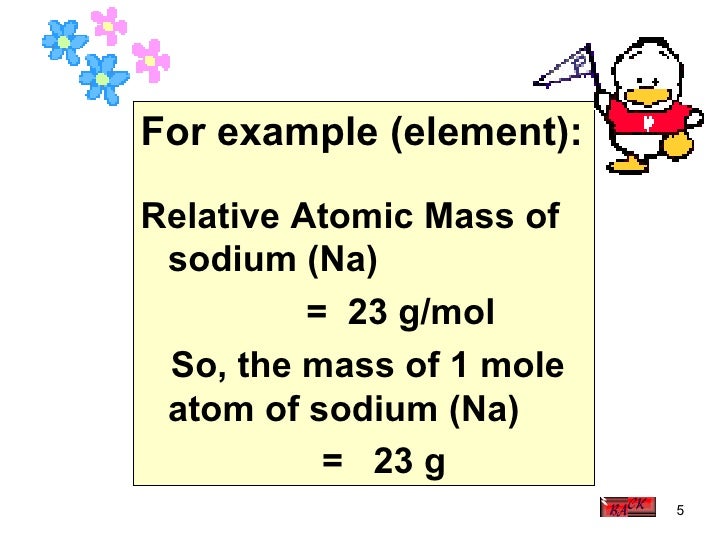 aE, bE, cE, etc, and with the respective abundances of A%, B%, C
aE, bE, cE, etc, and with the respective abundances of A%, B%, C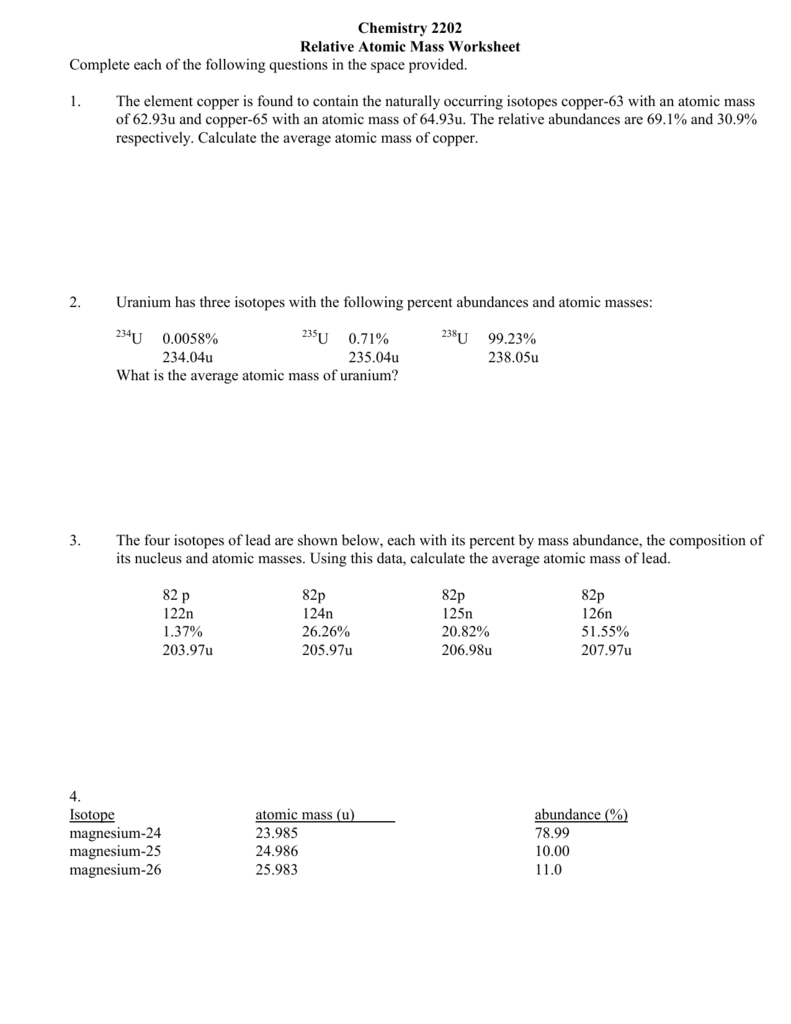 % etc,
% etc, | relative atomic mass (r.a.m.) | = ( | A 100 | × a) | + ( | B 100 | × b) | + ( | C 100 | × c) | + etc |
and 100 - x = %abundance of isotope-b
Relative Atomic Mass Formula
then, let r.a.m = relative atomic mass of the element:
| r.a.m. | = ( | x 100 | × mass isotope-a) | + ( | 100 - x 100 | × mass isotope-b) |
and solve for x

By international agreement the mass of an atom of carbon-12 is given as 12 unified atomic mass units (u). Therefore, 1 u is 1/12th the mass of an atom of carbon-12.
In most chemistry work, you can:
• neglect the tiny contribution to atomic mass from electrons
• take both the mass of a proton and a neutron as being equal to 1 u.
In a pure isotope all the atoms have the same atomic structure and the same mass.
Relative isotopic mass is then simply the mass number of the isotope, e.g. the relative isotopic mass of 32Sis 32.
However, most elements contain a mixture of isotopes, each with a different mass number. To find the relative atomic mass of an element we must know:
The relative isotopic mass of each isotope.
The proportion of each isotope in the element.
For example:
Copper has 2 isotopes, 63Cu and 65Cu. The proportions of each isotope are 69.17% and 30.83%.
These can be rounded to 70% and 30%.
The relative atomic mass of Copper is therefore (70/100 x 63) + (30/100 x 65) = 63.6
There is no unit as it is a relative value.
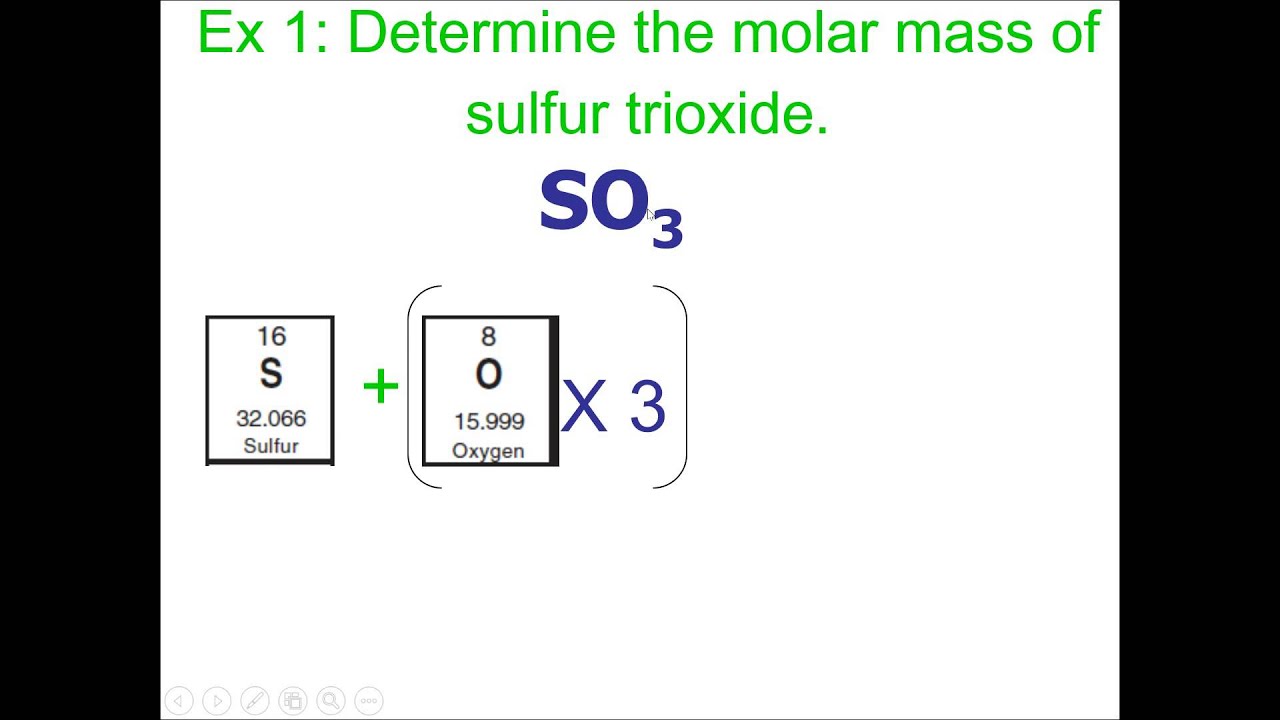
Molecular Mass (Mr) is the sum of all the relative atomic masses for all the atoms in a given formula.
For covalent compounds it is called the Relative Molecular Mass.
For ionic compounds it is called the Relative Formula Mass.

Methane (CH4)for example, contains 1 Carbon atom and 4 Hydrogen atoms.
Relative Atomic Mass Of Hydrogen
Therefore, the molecular mass is calculated as follows:
C = 12, H = 1
Relative Atomic Mass
CH4 = C + (H x 4) = 12 + (1 x 4) = 16
