His name was Amedeo Avogadro, not Amidio nor Amadeo nor Avegadro nor Avagadro. The full name was Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto. Avogadro's law states that. Equal volumes of all gases, at the same temperature and pressure, have the same number of molecules. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10 -19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single.
Not much is known about the infamous Avogadro besides his contributions to Chemistry and Physics. Avogadro was born on August 9th, 1776 as the son of the Count of Quaregna (a title he later inherited). Avogadro spent most of his life in his hometown of Turin, Italy. He later became a professor at the Turin University. Avogadro had six children with a loving wife, and was a strong religious man. He was said to be liked by his students because of his sense of humor also. Avogadro started college when he was only 13, and graduated when he was 16. Avogadro received his doctorates in ecclesiastical law (law pertaining to the church) at the ripe age of 20. Following that, he began to practice law. Although Amedeo Avogadro was destined to become a lawyer, his main interests changed to chemistry, mathematics, and physics.
In the year 1800, Avogadro began his private studies in Physics and Mathematics. In his free time he did a lot of reading and had a complete set of the current scientific journals in his library printed in four
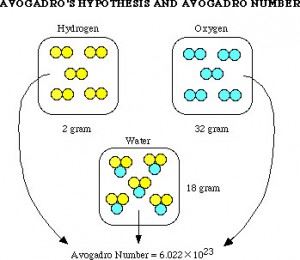 different languages. In one of these journals he read in 1808 that Joseph Louis Gay-Lussac, “Had found that when two gasses react together to form different products, the volumes of the reactants and the products (if they are all still gasses) are all whole numbers.” This accepted belief was called the Law of Combining Volumes. Avogadro then thought, hey, this must mean that equal amounts of gas at the same temperature and pressure must contain the same amount of molecules. He knew that when two volumes of Hydrogen and one volume of oxygen were reacted it formed two volumes of water vapor. Why two and not one? Avogadro’s answer was that it must mean that sets of two oxygen atoms were used to create the one volume of oxygen he used. After noticing this, Avogadro was able to see that, “Molecular weights of any set of gasses are the same as the ratio of the densities to those gasses under the same conditions of temperature and pressure.” His conclusion was that, “All gasses, simple or complex, contain the same number of molecules under the same conditions of temperature and pressure.” This idea is one of the main points in Chemistry. Although Avogadro did not literally find the unit of Moles (6.02 x 10^23 molecules), the number is called Avogadro’s Number in honor of his theories that led future scientists to be able to calculate this number.
different languages. In one of these journals he read in 1808 that Joseph Louis Gay-Lussac, “Had found that when two gasses react together to form different products, the volumes of the reactants and the products (if they are all still gasses) are all whole numbers.” This accepted belief was called the Law of Combining Volumes. Avogadro then thought, hey, this must mean that equal amounts of gas at the same temperature and pressure must contain the same amount of molecules. He knew that when two volumes of Hydrogen and one volume of oxygen were reacted it formed two volumes of water vapor. Why two and not one? Avogadro’s answer was that it must mean that sets of two oxygen atoms were used to create the one volume of oxygen he used. After noticing this, Avogadro was able to see that, “Molecular weights of any set of gasses are the same as the ratio of the densities to those gasses under the same conditions of temperature and pressure.” His conclusion was that, “All gasses, simple or complex, contain the same number of molecules under the same conditions of temperature and pressure.” This idea is one of the main points in Chemistry. Although Avogadro did not literally find the unit of Moles (6.02 x 10^23 molecules), the number is called Avogadro’s Number in honor of his theories that led future scientists to be able to calculate this number. The impact of this amazing realization is still spreading today. Avogadro’s law is used in thousands of different applications each and every day; from a high school chemistry I class, to scientists trying to figure out the cure to cancer. This number, and law is used to find the outcome of a reaction before it is even attempted, and is the basis of many theoretical chemistry and physics ideas. Although the applications for this are limited to chemical equations and the such, the combinations are endless, straying into the realm of the chemistry of DNA and other complicated molecular structures. We are only beginning to use this law to its full extent, and we will continue to broaden its impact by using it more.
Amedeo Avogadro was a bright man who stumbled upon a question chemists had encountered for years and somehow, with some great brain power, found the answer. The only dilemma with this historic find was that Avogadro’s life was, for the most part, isolated from other scientists. This was one of the main reasons why his theory did not catch on quickly. In fact, it was not until 1858 when the significance of Avogadro’s work was realized. When Italian chemist Stanislao Cannizzaro explained why Avogadro’s law did not always hold true in some rare instances, Avogadro’s hypothesis finally became the accepted theory at the time.
07th May 2019 @ 12 min read
The Avogadro constant or (the Avogadro number earlier) is the number of elementary units in one mole of any substance. The Avogadro constant is denoted as NA. It has the dimension of the reciprocal amount of substance (mol−1). The approximate value of NA is 6.022 × 1023 mol−1. This means one mole of any substance contains 6.022 × 1023 elementary particles. The Avogadro constant is named after Italian scientist Amedeo Avogadro.
These elementary units in one mole can be anything like atoms, molecules, ions, electrons, protons, neutrons, particles of sand. So, when we say one mole of sodium chloride, it means 6.022 × 1023 molecules of sodium chloride.
Values of Avogadro's Constant
The value of the Avogadro constant is revised over a period of time. As of the 2019 redefinition of the SI base units, the value of the Avogadro constant is fixed to 6.02214076×1023mol−1. This is the exact value of the constant. The table below mentions the value of the constant in different units.
| Value | Unit |
|---|---|
| 6.022 140 76 × 1023 | mol−1 |
| 6.022 140 76 × 1026 | kmol−1 |
| 2.731 597 099 802 001 2 × 1026 | lb-mol−1 |
| 1.707 248 187 376 250 75 × 1025 | oz-mol−1 |
| 0.602 214 076 | mL mol−1 Å |
/Avogadro-58f7d6f35f9b581d5983024e.jpg)
History of Avogadro's Constant
The Avogadro constant has a long history. The constant is named in honour of Avogadro, but he did not discover it. In 1811, Avogadro discovered the relationship between the volume of gas and the amount of gas through his experiments. He was the first to proposed the volume of a gas is directly proportional to the amount of the gas at constant pressure and temperature. This is today we call Avogadro's law or Avogadro's hypothesis. His work does not mention Avogadro's constant.
Perrin's Work
French Nobel Laureate Jean Baptiste Perrin estimated the Avogadro number with several methods. And he credited the naming of the number to Avogadro in 1909. Perrin named the number Avogadro's number, not Avogadro's constant. This name had continued till 1971. In 1971, the International System of Unit (SI) introduced a new quantity called Avogadro's constant. The Avogadro constant has the same numerical value as the Avogadro number, but they differ in the unit which will be explained later in this article.
Perrin defined the Avogadro number as the number of atoms in one gram of hydrogen (one gram-molecule). This definition was later revised to the number of atoms in 12 grams of carbon-12 (12C).
Loschmidt's Estimation
Before Perrin, Loschmidt also made a significant contribution to the number. Josef Loschmidt was an Austrian scientist who is notable for his work on estimation of the diameter of the molecules in the air. Through his method, it is possible to calculate the number density (the number of molecules or atoms per unit volume). This quantity is closely relative to the Avogadro constant. The relationship between them is discussed later in this article. The number density of an ideal gas is called as the Loschmidt constant. In many of German literature, these two constants are interchangeable. They can easily be distinguished from their units. The Avogadro constant is also denotated as L in honour of Loschmidt.
Other Efforts
Robert Millikan was an American physicist and Nobel Laureate. He successfully measured the charge on an electron in 1910. The electric charge per mole (the Faraday constant) of electrons had already known at that time. With the help of these two quantities, the electric charge on an electron and the Faraday constant, it is possible to calculate the number of electrons per mole. The value of this number of electrons per mole is the same as Avogadro's constant.
One of a modern method to estimate the value of the constant is X-ray crystallography. This method estimates the constant by determining the number of silicon atoms in a crystal cell, the volume per unit cell, and the molar volume.
The measurement of the accurate value of the Avogadro's constant is always troublesome. Over the period, the new methods were developed, and the Avogadro constant has continuously been improvised. From 2019, the international committee fixed the value of the Avogadro's constant exactly to 6.022 140 76 × 1023 mol−1.
2019 Redefinition and Prior Definition of Avogadro's Constant
As discussed above, the 2019 redefinition of the Avogadro constant is 6.022 140 76 × 1023 mol−1. The consequence of this redefinition is the prior definition of the constant is no longer valid. Before the 2019 redefinition, the value of the constant was defined as the amount of atoms presents in 12g of carbon-12 (12C). Also, because of the definition the molar mass constant (Mu) is no longer exactly equal to 1 g mol−1. Instead, it is approximately equal to 1 g mol−1. This is summarised in the table below.
| 2019 Redefinition | Prior to 2019 Redefinition |
|---|---|
| NA = 6.022 140 76 × 1023 mol−1 | The value of NA is the number of 12C atoms in 12 g of carbon-12. |
| The molar mass constant is approximately equal to 1 g mol−1 (Mu ≈ 1 g mol−1). | Mu is exactly equal to 1 g mol−1 (Mu = 1 g mol−1). |
Note: The difference in the value of the Avogadro constant before and after the 2019 definition is very small. The redefinition would not affect most of the calculations unless the high degree of precision is needed. For practical calculations, we can take NA = 6.022 × 1023 mol−1.
Avogadro's Constant and Mole
The Avogadro constant and the mole are related quantities. In fact, the Avogadro constant is defined in terms of the mole. The value of Avogadro's constant is the number of elementary units in one mole of any substance. The definition is universally true. The below equation establishes the relation between both.
Avogadro's Constant and Molar Mass
We can use the Avogadro constant to determine the mass of any atom if we know the molar mass of that atom. This statement is also true for molecules. The molar mass is the mass of one mole of a given sample. It is expressed in g mol−1. The relation between both is as follows:
where mi is the mass of atom i and Mi is molar mass of atom i.
Avogadro's Constant and Avogadro's Number
The Avogadro constant and the Avogadro number have the same numerical value. They only differ in the unit. The Avogadro number is a dimensionless quantity, but the Avogadro constant has the dimension of the reciprocal amount of substance (mol−1). The below table describes the same.
| Avogadro's Constant | Avogadro's Number |
|---|---|
| The constant has the unit of mol−1. | It is a dimensionless quantity. |
| It is denoted as NA. | We use N to denote the Avogadro number. |
| NA = 6.022 × 1023 mol−1 | NA = 6.022 × 1023 |
Avogadro's Constant and Boltzmann's constant
The Boltzmann constant is an important physical constant which plays a vital role in classical statistical mechanics. It is denoted as kB or simply k. The Avogadro constant is related to the Boltzmann constant by the gas constant R.
Avogadro's Constant and Loschmidt's Constant
The Loschmidt constant is the number density (the number of molecules per unit volume). For an ideal gas, the relationship between the Loschmidt constant and the Avogadro constant at STP (P0 = 1 atm, T0 = 273.15 K) is described in the equation below.
Avogadro's Constant and Faraday's Constant
The Faraday constant (F) is the Avogadro constant times the elementary charge (e).
Avogadro's Constant and Unified Mass Unit
The unified mass unit or the dalton (u) is the ratio of the molar mass constant (Mu) and the Avogadro constant.
where mu is the atomic mass constant.
The value precise value of Mu is 0.999 999 999 65(30) g mol−1. But for practical purposes, we can say Mu ≈ 1 g mol−1.
Examples
Example 1: To Determine Calcium Atoms
Statement: For 100 g of calcium in a beaker, calculate the number of calcium atoms in the beaker?
Solution: The molecular weight of calcium is 40.1 g mol−1. The number of moles of calcium in the beaker is
The number of calcium atoms in the beaker is calculated as:
Therefore, the number of calcium atoms is 1.50 × 1024.
Example 2: To Determine Total Molecules in Sodium Chlorine Solution
Statement: Consider 50.0 g of NaCl is dissolved in 200 g of water. Estimate the total molecules in the solution?
Solution: The molecular weight of NaCl and water is 58.44 g mol−1 and 18.01 g mol−1.
The moles of NaCl in 50.0 g:

The moles of H2O in 100 g:
When 1 mol of NaCl dissociates, 1 mol of Na+ and 1 mol of Cl− are formed. So, when 0.855 5 mol of NaCl dissociates, 0.855 5 mol of Na+ and 0.855 5 mol of Cl− are formed.
$underset{1,text{mol}}{ce{Na+}}$ + $underset{1,text{mol}}{ce{Cl-}}$}' alt='>Thus, the total number of moles after the dissociation is the sum of the moles of Na+, Cl−, and H2O.
The total number of molecules in the solution is
Therefore, the total number of moles in NaCl solution is 4.374 × 1024 mol.
Example 3: To Determine Mass of Sodium Atom
Statement: The molar mass of sodium-23 is 22.989 g mol−1. Calculates the mass of a sodium atom?
Solution: Let mNa and MNa be the atomic mass and molar mass of sodium-23. Thus, MNa = 22.989 g mol−1.
Now, mNa can be determined using the formula below.
Avogadro's Number Problems
Therefore, the mass of a sodium-23 atom is 3.817 × 10−23 g.
Example 4: To Determine Molecular Mass of Iodine gas
Statement: The atomic mass of iodine is 126.9 g mol−1. Determine the molecular mass of iodine gas?
Amedeo Avogadro Constant
Solution: The iodine gas is a diatomic gas. The molecular formula is I2. So, the molar mass of I2 is twice the molar mass of I.
Now, mI2 can be determined as:
Therefore, the mass of a iodine molecule is 4.208 × 10−22 g.
Associated Articles
If you appreciate our work, consider supporting us on ❤️Avogadro's Number Example
patreon.Avogadro's Law Formula
- 4
- cite
- response
Copy Article Cite
29th Oct 2019
